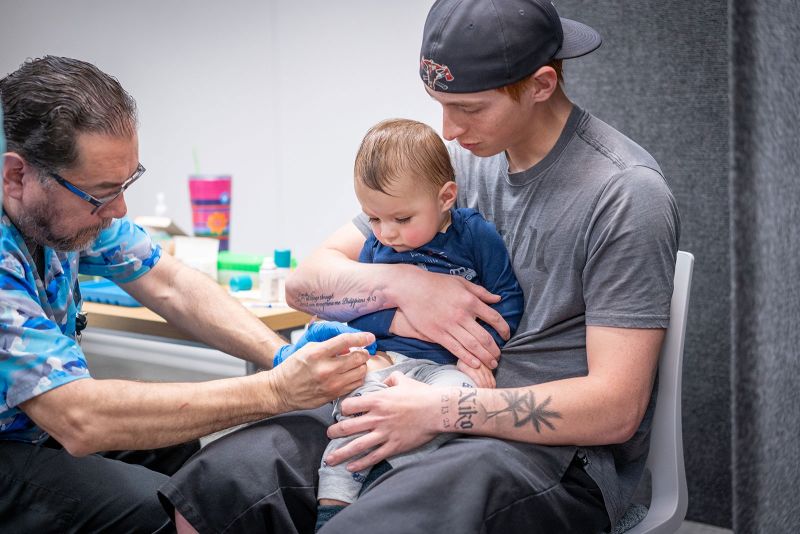
FDA may ask Novavax to conduct additional trials of its Covid-19 vaccine to receive full approval
The terms need to be negotiated before Novavax’s vaccine could be granted full approval, the source said, declining to be named because they weren’t authorized to speak on behalf of the FDA.
“We can confirm we have responded to the FDA’s Post Marketing Commitment (PMC) request and are awaiting feedback from the agency,” Silvia Taylor, executive vice president and chief corporate affairs and advocacy officer at Novavax, said in a statement Friday. “PMCs are not unusual with many approved drugs / biologics having at least one PMC or requirement. We continue to believe that our application is approvable, and we look forward to our continued engagement with the FDA about their request for a PMC and to moving to approval as soon as possible.”
A spokesperson for the US Department of Health and Human Services, the FDA’s parent agency, said Friday that it “remains committed to our promise: ensuring products are safe for the American people and grounded in gold-standard science.”
The Novavax Covid-19 vaccine, which uses more traditional protein-based technology than the newer mRNA vaccines from Pfizer/BioNTech and Moderna, has been subject to emergency use authorization since 2022. But with FDA action, it would be the third vaccine against Covid-19 to receive full FDA approval, which could provide additional reassurance to people seeking the shot.
The missed deadline came at the same time the FDA named Dr. Scott Steele acting director of the Center for Biologics Evaluation and Research, which oversees vaccine regulation, days after former director Dr. Peter Marks was forced out. In his resignation letter, Marks cited “efforts being advanced by some on the adverse health effects of vaccination” that he called “concerning.”
HHS Secretary Robert F. Kennedy Jr., a longtime anti-vaccine advocate, has falsely called vaccines for Covid-19 “the deadliest vaccine ever made” and more recently made misleading statements about the safety of the measles vaccine amid a deadly outbreak centered in West Texas.